Consider Pharmacogenetics When Choosing an Antidepressant or Anxiolytic
After a long ER shift, you are wrapping up your day when a patient comes in and complains of “feeling off.” When you ask about her symptoms, she describes several episodes of feeling lightheaded and the sense that her heart is “racing.” Her husband adds that she has recently had a fainting episode while at the gym and another at the grocery store. She adds that, prior to these episodes, she experienced some weakness and blurring of vision. In order to differentiate your diagnosis between Long QT syndrome and other conditions, you inquire about the duration of her symptoms. She indicates that her symptoms started around the time of her recent depression diagnosis. She states that she originally suspected her symptoms were due to the severity of her depression.

You order lab tests and an EKG and review her medication list, which indicates she is currently taking citalopram to treat her depression. You remember that citalopram has been linked to acquired Long QT syndrome. When the EKG comes back, you confirm the presence of Long QT syndrome and are unable to find any alternative explanation for the development of her syndrome. Once the patient is stabilized, you refer her to her cardiologist and psychiatrist in order to safely discontinue her citalopram. You also suggest pharmacogenomic testing for further evaluation. The results of the patient’s PGx testing reveals she is a CYP2C19 Poor Metabolizer (PM), which affects her ability to effectively metabolize the citalopram prescribed for her depression. Specifically, CYP2C19 PMs should not be prescribed more than 20mg/day citalopram to prevent QT elongation.1 The patient’s psychiatrist initiates her on a more appropriate medication based on her PGx testing results.
Treatment for anxiety and depression can be complicated: patients are often less compliant with their prescriptions if they develop a tolerance to or troublesome side effects from a medication. Fortunately, PGx testing can greatly reduce the length of time it takes to find a suitable medication by focusing on the efficiency of the enzymes responsible for drug metabolism. Many of the medications used to treat anxiety and depression are metabolized through the cytochrome p450 family of enzymes and individual variations in the efficiency of these enzymes can result in altered medication metabolism. A particular drug or dosage may be inappropriate for an individual that metabolizes that drug at a higher or lower rate than normal. The following table lists several drugs or drug classes commonly prescribed for depression and anxiety and the genes/biomarkers currently associated with the metabolism of that drug or drug class.
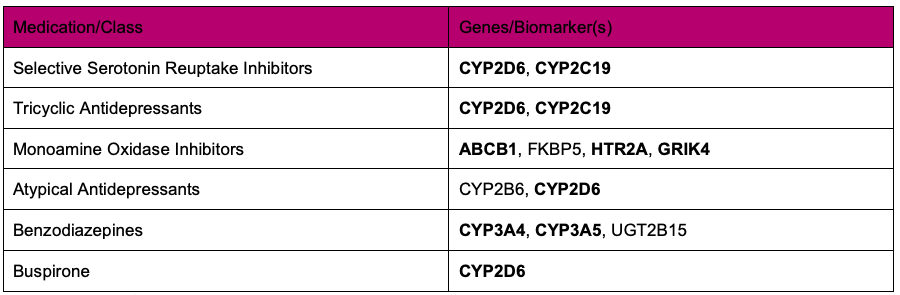
Table: Examples of antidepressants and anxiolytics and the gene/biomarker involved in metabolizing the respective medication or medication class.2 The patient metabolizer status is reported for all of the genes in bold type in the Kailos Genetics InspexionTM v.2.4 testing panel.
The simplest way to evaluate the metabolizer status of a patient is through pharmacogenetic testing. The Kailos Genetics InspexionTM pharmacogenetic test reports the metabolizer status of key pharmacogenetic genes, including CYP2C19 and all of the genes in the above table listed in bold type, making it one of the most comprehensive panels in the industry. We are a CLIA-certified lab that utilizes next generation sequencing (NGS) in concert with our TargetRichTM proprietary workflow to produce high quality sequences with unparalleled accuracy.

We extract DNA from a buccal cell collection kit that can be performed on-site or by the patient at home to make testing easy, simple and painless. Testing is completed in about two weeks, and results are released to the physician to guide future treatment. If test interpretation assistance is required, Kailos Genetics has partnered with genetic counseling service providers to facilitate that relationship. Kailos Genetics empowers physicians by enabling them to customize drug therapies to each patient’s unique physiology.