What are the Differences Between Various COVID Tests?
December 15, 2020
There is an awful lot of terminology floating around these days when it comes to COVID-19 testing. And with more than 200 different tests currently approved by the Food and Drug Administration (FDA) for the detection of COVID-19 (COVID),1 understanding the differences between various tests can be difficult.
Here, we provide a brief guide for deciphering some of the terminology associated with different types of COVID testing and additional information summarizing the purposes, effectiveness and accuracy of these testing methods.
There are three basic types of COVID tests: antibody, antigen and molecular-based. COVID is caused by infection with the SARS-CoV-2 (CoV-2) virus. The three types of COVID tests differ in which molecules associated with CoV-2 infection they detect, and because of this, the three types of tests are best suited for different parts of the CoV-2 infection cycle. The molecules detected by each test are listed below.
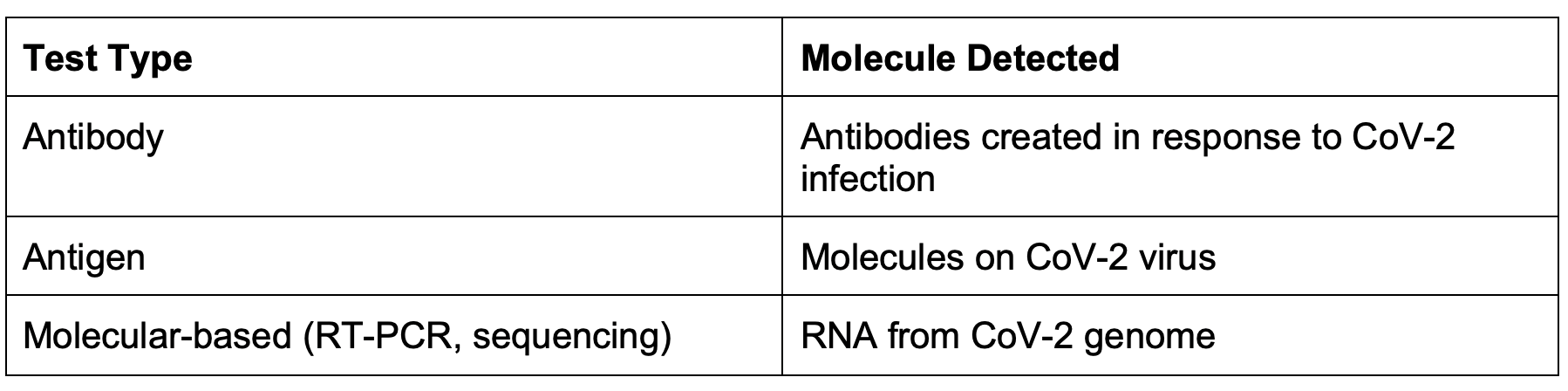
Antigen and molecular-based tests are used to detect active CoV-2 infection in different ways. Molecular-based tests use methods such as reverse-transcription polymerase chain reaction (RT-PCR) and/or DNA sequencing to detect even the slightest amounts of CoV-2 genome, making molecular tests highly sensitive and capable of detecting CoV-2 infection at the earliest possible point in the infection cycle. Molecular-based tests usually take between one to two days to complete. Antigen tests, while still detecting active CoV-2 infection, instead detect molecules found on the virus itself. Many different rapid-antigen tests have been developed to detect CoV-2 in a matter of minutes; however, the sensitivity of these tests lags behind molecular-based tests and is usually best suited for testing symptomatic individuals.2 Antibody COVID tests, on the other hand, detect antibodies that the immune system has produced against the CoV-2 virus. Because antibody production occurs later in the infection cycle, antibody tests are best suited for detecting past CoV-2 infection and take approximately three days to receive results.
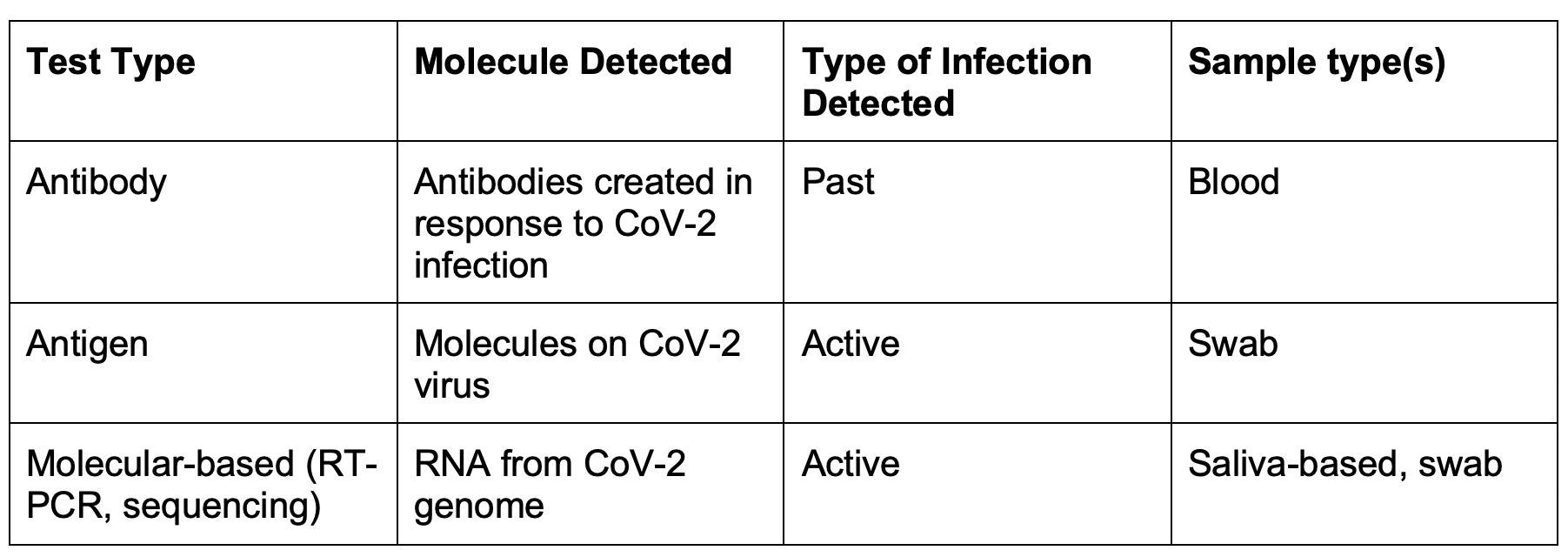
The different types of COVID testing can also differ in what samples are collected for testing. There are three sample types that are commonly used for COVID testing: saliva-based, swab and blood. Swab samples are commonly used for COVID testing and include nasal, throat and nasopharyngeal swabs. The swabs collect secretions that contain CoV-2 in a COVID-positive individual. Saliva-based samples include oral rinse or saliva collection kits to detect CoV-2. Both swab and saliva-based samples are used to identify active CoV-2 infection. Currently, only molecular-based tests are used to detect CoV-2 in saliva-based samples. In contrast, blood samples are used to detect CoV-2 antibody production in individuals that have recovered from past CoV-2 infection. A summary of the sample types used for each type of COVID test is included in the table below.
So with all of the COVID testing and sample types available, which combination is the most accurate?Unfortunately, large, well-controlled and peer-reviewed studies comparing different COVID testing methods and sample types have not been conducted yet. A smaller, preliminary study performed in the healthcare setting suggests that saliva and nasopharyngeal swab samples are comparable when paired with RT-PCR for CoV-2 detection.3 Between the three types of COVID tests, one metric used to quantify the sensitivity of a test is the false-negative rate, or the rate at which the test fails to detect CoV-2 in an individual that is COVID-positive. While the false-negative rates for tests vary widely between preliminary studies, the highest false-negative rates we observed for molecular-based tests was up to 40%,4,5 for antigen tests was up to 68%,2 and for antibody tests was up to 8%.6
Molecular-based tests have been considered the most sensitive of the active CoV-2 infection testing types, and over time, false-negative results have been reduced by waiting to test individuals exposed to COVID until at least five days after the close contact with a COVID-positive person occurred.7 This waiting period ensures that, if infected, an individual’s viral load (amount of virus present in the body) is high enough at the time of testing to produce an accurate result. Additionally, some antigen tests are only approved for testing symptomatic individuals,2 who presumably have a higher viral load than presymptomatic individuals. Rapid-antigen tests remain popular for their quick testing turnaround time compared to the one to two days required for molecular-based tests.
When determining which COVID test and sample type is right for your application, there are a variety of questions to consider, including:
● Do you need to detect active or past infection?
● Do you need to detect symptomatic or presymptomatic COVID cases or both?
● Are you assessing overall infection rate or trying to prevent a COVID outbreak?
● Is there a shortage of swabs for sample collection?
Each testing method and sample type has their own benefits that must be weighed against drawbacks. And while certain metrics can be known now with some certainty, such as testing turnaround time and costs, the accuracy of testing performed in the field will be better established in the future. Kailos Genetics has designed an economical and fully-customizable COVID testing platform, Assure SentinelTM, that allows organizations of any size to operate safely during the pandemic. Click here to learn more about the program or contact us with any questions you may have regarding our COVID screening services.
1U.S. Food and Drug Administration. In vitro diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
2Katherine J. Wu. A rapid test falters in people without symptoms, study finds. The New York Times. https://www.nytimes.com/2020/11/02/health/coronavirus-testing-quidel-sofia.html
3Wyllie A, et al. Saliva and nasopharyngeal swab specimens for the detection of SARS-CoV-2. New England Journal of Medicine. N Engl J Med 2020; 383:1283-1286. https://www.nejm.org/doi/full/10.1056/NEJMc2016359
4Fang Y, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. Feb 19, 2020. https://pubs.rsna.org/doi/10.1148/radiol.2020200432
5Woloshin S, et al. False negative tests for SARS-CoV-2 infection--challenges and implications. New England Journal of Medicine. Aug 6, 2020. https://www.nejm.org/doi/full/10.1056/NEJMp2015897
6Deeks JJ, et al. What is the diagnostic accuracy of antibody tests for the detection of infection with the COVID-19 virus? The Cochrane Collaboration. https://www.cochrane.org/CD013652/INFECTN_what-diagnostic-accuracy-antibody-tests-detection-infection-covid-19-virus
7Minnesota Department of Health. COVID-19 testing. https://www.health.state.mn.us/diseases/coronavirus/testsites/index.html Accessed November 27, 2020.